Biostatistical Programming Services at NxtGen’s
Professional statisticians
Biostatistical Programming Service
NxtGen’s offers comprehensive Biostatistical Programming Services designed to support researchers in medical, life science, and healthcare sectors with advanced statistical analysis and data management. Our expert team provides precise statistical programming solutions to ensure rigorous analysis and interpretation of research data.
Our Case Report Rewriting Services Include
✅ Advanced Statistical Analysis: Clinical data management services utilize state-of-the-art statistical techniques and software to conduct thorough analyses, from basic descriptive statistics to advanced inferential modeling by clinical trial solutions.
✅ Data Management Expertise: According to regulatory clinical research, health services research biostatisticians handle diverse datasets with precision, ensuring accuracy, integrity, and compliance with industry standards through meticulous data cleaning and transformation.
✅ Tailored Programming Solutions: Clinical data management services develop customized statistical algorithms and analysis scripts, health services research and clinical research services, working closely with researchers to implement models that align with specific study objectives as regulatory clinical research.
✅ Regulatory Compliance: Clinical data management services analyses strictly follow guidelines set by regulatory bodies such as the FDA and EMA, ensuring seamless clinical trial submissions.
✅ Comprehensive Reporting: Clinical data management services deliver detailed statistical reports and visualizations, providing clear insights for decision-making, research publications and stakeholder presentations.
Why Choose NxtGen’s Biostatistical Programming Services?
With a commitment to accuracy, reliability, and regulatory compliance, clinical data management services ensure your regulatory clinical research findings are robust and credible. Contact us today to discuss your biostatistical needs and elevate your research outcomes!
How a physician Manuscript Service Works
Our step-by-Step Process
1
Consultation :
A detailed discussion to understand your specific requirements, research goals, and target audience for a customized manuscript approach.
Step - 1
next step
2
In-Depth Research :
Extensive literature review and data collection to ensure a well-informed, evidence-based, and scientifically accurate manuscript by clinical data management services.
Step - 2
next step
3
Manuscript Writing :
Expertly structured content crafted with precision, ai data analysis software adhering to medical writing standards and journal guidelines.
Step - 3
next step
4
Rigorous Review
Thorough quality checks for clarity, coherence, and compliance with industry standards before final submission.
Final
Getting Started With This Service is Easy!
Streamlined adoption of CDISC standards to enhance regulatory approval and data standardization.
Advanced statistical methodologies ensuring precision and credibility in research findings.
Transform complex data into actionable insights with cutting-edge analytical techniques.
End-to-end support for clinical trials, ai data analysis software from study design to regulatory submission.
Data-driven statistical evaluations to support clinical and medical research.
Secure and efficient handling of research data to ensure compliance and accuracy.
Dedicated experts managing timelines, deliverables, and research workflows efficiently.
Study design, data management, and statistical analysis for optimal research outcomes.
Advanced EHR solutions on health services research for accurate data collection, organization, and analysis in clinical research.
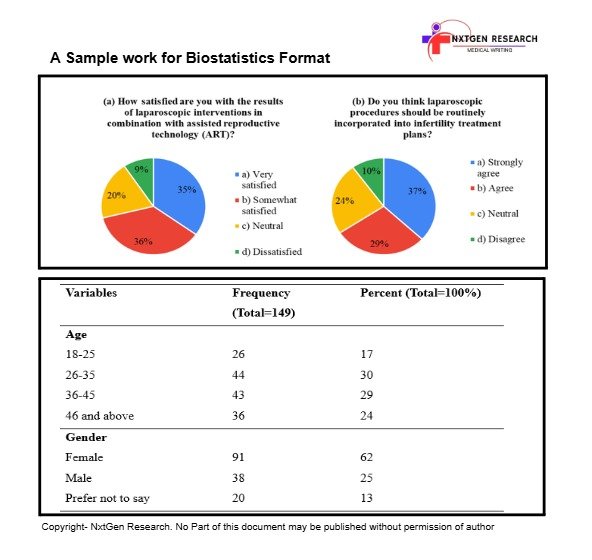
Biostatistical Programming Services Sample Work
NxtGen’s services streamline the process, ensuring your research meets rigorous publication standards swiftly
Our Commitment to Excellence
Guaranteed Quality
- If our work doesn’t meet your expectations, we’ll revise it until you are fully satisfied. Your success is our priority.
- We ensure precise, impactful outcomes in medical research through our commitment to excellence and accuracy.
Always On Time
- We meet deadlines efficiently, often delivering work within 8 hours to keep your projects on schedule.
- NxtGen ensures timely completion of projects, maintaining workflow efficiency and reliability at every stage.
Enhanced Security
- We safeguard your data with top-tier encryption, strict confidentiality policies, and non-disclosure agreements for all team members.
- Your research is secure with us, as we enforce rigorous protocols to maintain privacy and integrity.
Our Biostatistical Expertise Covers:
- Expert Statistical Consulting – Providing comprehensive support for drug and medical device development by clinical research services.
- Clinical Trial Randomization – Ensuring unbiased study outcomes through effective randomization techniques.
- Precise Sample Size & Power Calculations – Optimizing study design with statistically sound calculations by clinical research services.
- Comprehensive Statistical Reports – Crafting detailed statistical analysis for clinical study reports and research manuscripts.
- Integrated Efficacy & Safety Summaries –Clinical trial data management offering in-depth evaluations of treatment effectiveness and safety profiles.
- Data Safety Monitoring – Supporting safety review boards with statistical expertise and regulatory insights.
- Advanced Statistical Methodologies – Utilizing pooled analyses, meta-analyses, data analytics education and other multifaceted statistical techniques.
- Health Economics & Comparative Research – Delivering insights into cost-effectiveness and treatment impact.
- Protocol Development & Evaluation – Providing thorough statistical input for clinical study designs.
- Comprehensive Statistical Analysis Plans (SAPs) – Designing structured SAPs with detailed table layouts for accurate data interpretation.
SCRIPTING SUCCESS STORIES


Frequently Asked Questions
Get expert help at every turn—our FAQs address your toughest questions, and if you still need help, just give us a call!
We as a clinical trial data management offer comprehensive formatting services, data and analysis including citation styling, manuscript structuring, data analysis program and journal-specific formatting to meet publication requirements.
You will receive a professionally formatted document adhering to style guidelines, ensuring consistency, clarity, and compliance with journal specifications.
Statistical programming provide various packages, from basic formatting to advanced layout structuring, tailored to different academic and medical publishing needs.
Key details include research objectives, preferred sources, study scope, formatting guidelines, and any specific journal or institutional requirements.
Statistical programming team consists of experienced professionals with advanced degrees in medicine, science, and academic writing, ensuring high-quality, expert content clinical research services.
Once you order, we review requirements, conduct research, draft the review, refine content, and deliver a structured, high-quality document.
Yes, we provide revisions and, if necessary, refunds based on our satisfaction guarantee policy, clinical trial data management ensuring you receive a quality document.
Clinical trial data management guarantee adherence to guidelines, timely delivery, quality assurance, confidentiality, and unlimited revisions to meet your expectations.
No, all projects are handled in-house by our expert team, ensuring quality, data and analysis, confidentiality, and adherence to the highest professional standards.

